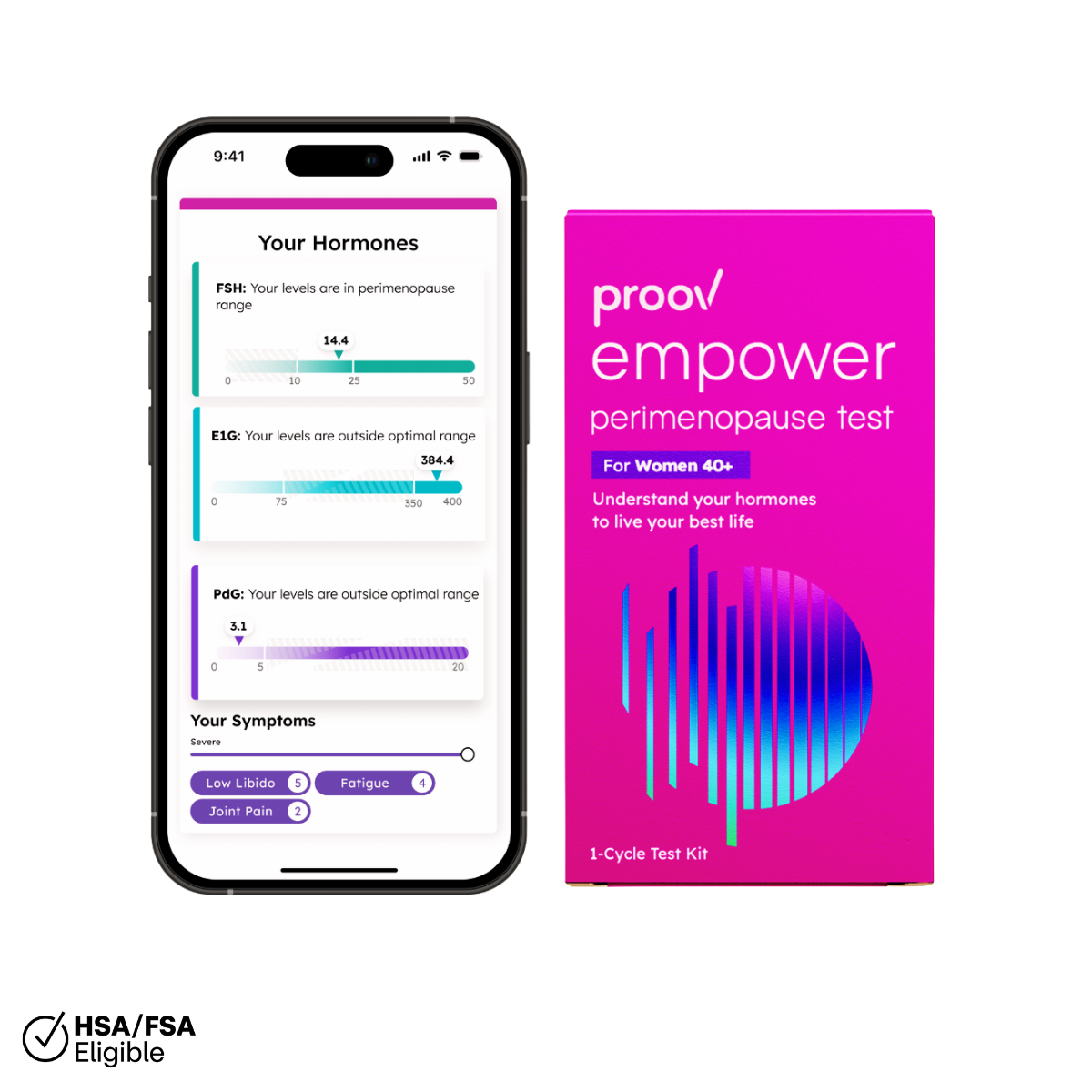
Over the past several months, we have all been working hard alongside the FDA and are so excited that as of about a week ago, Proov is FDA cleared! We’ve learned SO much through this process and are thankful for the efforts of the entire Proov team. After all, it’s not every day you have an opportunity to work with the FDA.
The FDA is the Food and Drug Administration, an agency within the U.S. Department of Health and Human Services that protects the public health by assuring the safety, effectiveness, and security of drugs, vaccines, and medical devices. To support its mission of ensuring U.S. consumer safety, the FDA reviews the accuracy of a device and clears it if they deem it to be substantially equivalent to the safety and performance attribute of a device that was previously cleared by the FDA.
What is “FDA Cleared?”
Getting FDA clearance means the FDA has reviewed scientific research and has indirectly agreed the product applied is accurate, safe, and effective for its intended use. In the case of Proov, our intended use – in other words the information we want Proov to provide to women – is that it confirms ovulation. Since problems with ovulation make it difficult (almost impossible) to successfully conceive, this is crucial when trying to get pregnant. In fact, the underlying cause of Amy’s infertility was a luteal phase defect, which is a problem with ovulation – and the ultimate reason she invented Proov.
Why isn’t Proov “FDA Approved”?
This is a question we get often and an important one! The term “FDA Approved” is reserved for the highest risk devices – think pacemakers, surgical implants, prescription drugs, vaccines, and for innovative products (those with no predicate device), neither of which apply to our product. In the case of almost all of these devices, the FDA must use a higher bar to ensure safety and efficacy because if the devices or drugs have deficiencies the risk of injury or worse is high.
In the case of Proov, our devices are considered to be of lower risk.
Proov’s FDA Clearance
Before Amy launched the first version of Proov (which back in the day was called “Ovulation Doublecheck”), she registered the product with the FDA, as is required by law.
The Proov PdG test technology had been previously validated as an accurate way to confirm ovulation. Due to our recent FDA clearance, we will be updating our website and packaging. Rest assured, the Proov test will remain the same effective, accurate test and our team will continue to provide all the support we can!
Other Fertility Tests: FDA Cleared
ClearBlue received its FDA clearance for predicting ovulation by measuring luteinizing hormone (LH) and estrogen.
Ovusense also has its clearance for predicting ovulation using basal body temperature tracking.
YO Sperm test , on the male side of the house, has also been cleared as a test to measure motile sperm.
If a test or device isn’t FDA cleared, does that mean it's unsafe?
Not necessarily. However, we think it is important for women to know the difference so they can make the most informed decision possible when selecting fertility products. Fertility products do concern one of life’s most important events, after all!
Only FDA cleared or approved products have been officially and independently reviewed and given a thumbs up by the FDA. If a device is only registered it does not mean anything with respect to the safety or performance of a product. Think of this like taking a test at school: you studied all week and think you aced the test, but you don't actually know the accuracy of your answers until you get your teacher (or the FDA in the case of medical devices) to evaluate them.
More questions for us? Shoot us a note! info@proovtest.com